Many people recognize the critical influence of DNA on genetic activity. However, some changes may affect how our genes work without affecting our DNA sequences. This is the field of epigenetics, and it is one of the most interesting new fields in studying how genetic activity works. Despite the promising research in this field, not many people know what epigenetics is or how it affects us.
 Epigenetics literally means “on top of or in addition to genetics,” and it refers to heritable traits that are not a result of DNA sequence. In simple terms, epigenetics is the study of heritable changes in gene expression without changes to the underlying DNA sequence.
Epigenetics literally means “on top of or in addition to genetics,” and it refers to heritable traits that are not a result of DNA sequence. In simple terms, epigenetics is the study of heritable changes in gene expression without changes to the underlying DNA sequence.
Gene expression is the process by which instructions in our DNA are converted into a functional protein. This process involves two primary sequential steps, known as transcription and translation. Transcription converts DNA to RNA, while translation converts the resultant RNA into protein.
Generally, changes in the functional proteins made are due to changes in the DNA sequence. However, epigenetic changes can regulate the turning “on” and “off” of genes. This results in an alteration in gene expression and protein production without any genetic change. This regulation ensures that cells produce only the functional proteins they need.
From this explanation of epigenetics, it is possible to define epigenetic traits. An epigenetic trait is a stably heritable phenotype that results from changes in a chromosome but without a change in the DNA sequence.1 While this definition is accepted in the scientific world, there have been alternate definitions that some scientists use.
The definition of an epigenetic trait reinforces the idea that epigenetics involve a change in a person’s phenotype without any corresponding change in their genotype.
There are different ways epigenetic changes affect gene expression. These types of epigenetic changes are also known as epigenetic modification mechanisms or epigenetic tags. These mechanisms form a layer of control within a cell that helps regulate gene expression and silencing.2 This regulation is essential in the differentiation of cells and the production of cells with specific functions.
There are three main epigenetic mechanisms, and they are as follows:
DNA methyltransferase enzymes mediate this epigenetic mechanism at cytosine-guanine sequence (CpG) sites. It involves transferring a methyl group to the C5 position of the cytosine to form 5-methylcytosine. This addition serves as a “chemical cap” and actually prevents the expression of certain genes and the subsequent formation of functional proteins.
If there is a need for the genes to be expressed, the chemical cap is removed in a process called demethylation. Although DNA demethylation is a process that is essential to development, it is less understood than methylation.
Currently, DNA methylation is regarded as the primary epigenetic modification. Scientists even suggest that it may be crucial to long-term memory function.3 The regulation of DNA methylation is key to normal cognitive function, and impairments to this mechanism can result in developmental mutations and be due to environmental risk factors like drug exposure.
Histones are proteins that provide structural support for the chromosomes and help control gene activity. Without histones, it would be impossible for DNA strands to fit inside cells as they would be too long. Histones wrap around the DNA, and if they wrap too “tightly,” cells cannot read the DNA. At this stage, the gene can be said to be turned “off.”
 Modifying the chemical groups on histones – by addition or removal of these groups – can loosen these histones and make DNA more accessible to proteins that read genes. When this happens, the gene is turned “on,” and it can be read.
Modifying the chemical groups on histones – by addition or removal of these groups – can loosen these histones and make DNA more accessible to proteins that read genes. When this happens, the gene is turned “on,” and it can be read.
Scientists have discovered at least nine types of histone modifications: acetylation, methylation, phosphorylation, ubiquitylation, GlcNAcylation, citrullination, crotonylation, sumoylation, and isomerization.4 The first four of these modifications have been well-researched, while the latter five are more recent discoveries. All of these modifications are enzyme-catalyzed.
Of all histone modifications, acetylation is the most straightforward. Other histone modifications are complex and often depend on DNA molecules in their surroundings.
This is the most recent discovery of the broad range of epigenetic changes. RNA can either be coding or noncoding in nature. If it is a coding RNA, it can make functional proteins. However, noncoding RNA is transcribed but not translated. For gene expression to occur, both transcription and translation are necessary.
Noncoding RNA and some other proteins attach to coding RNA and break it down so it does not make functional proteins. This process is also known as gene silencing or RNA silencing. It can occur at the transcriptional or post-transcriptional levels of gene expression.
These noncoding stretches of RNA can act as gene switches, as with other types of epigenetic modification mechanisms.5
There are different epigenetic influences on humans. These influences on humans come from different sources in the environment. Some of these influences are beneficial, while others are detrimental. The beneficial influences can improve the quality and length of life, while the detrimental ones can create an imbalance in the body and mind. This imbalance may manifest as psychological conditions and disorders.
The top three influences for epigenetics in humans are exercise, microbiome, and alternative medicine. In contrast, the undesirable influences are exposure to drugs of abuse and other toxicants.
Different factors affect epigenetic changes and determine whether or not an influence will be positive or negative. These factors include age, diet, seasonal changes, lifestyle, social interactions, environment, psychological state, health conditions, and financial status. All of these factors are a consequence of the environment, and the unique epigenetic and genetic profile of each individual influences the specific responses to these modulators.5
Epigenetics is not a theoretical concept without real-life applications, as it links with different day-to-day activities. Below are some real-life examples of epigenetics.
There is scientific evidence that suggests that sleep loss impacts the epigenome, which is the complete description of all the chemical modifications to the DNA and histone proteins of an organism. Further, scientists believe the resulting epigenetic changes to the epigenome may mediate the cognition alterations observed after sleep disruption or deprivation.6
While the evidence of the link between sleep and epigenetics has only recently come into the limelight, there is clear evidence that epigenetic changes occur after sleep deprivation.6 Therefore, epigenetic changes are a relevant prospect in diagnosing and managing sleep deprivation and disturbances.
 Aging is one of the most researched areas in relation to epigenetic alterations, and stress has a major role to play in aging. Studies show that cumulative lifetime stress can speed up the rate of epigenetic aging.7 The type of stress in question is chronic stress. Aside from its role in epigenetic aging, stress can also cause novel DNA modifications (N6-methyladenine) in the brain that can cause different neurological conditions.8
Aging is one of the most researched areas in relation to epigenetic alterations, and stress has a major role to play in aging. Studies show that cumulative lifetime stress can speed up the rate of epigenetic aging.7 The type of stress in question is chronic stress. Aside from its role in epigenetic aging, stress can also cause novel DNA modifications (N6-methyladenine) in the brain that can cause different neurological conditions.8
Chronic stress is quite common in today’s world, with everything occurring at a rapid pace. Chronically stressed people may even sometimes be regarded as “strong.” The body has a system that helps manage chronic stress; it is known as the NeuroEndoMetabolic (NEM) Stress Response. This system consists of six circuits that span several body organs, aiming to regulate stress levels in the body.
When the stress levels get too high, the body’s cortisol demands increase. This hormone is popularly known as the stress hormone. The adrenal glands produce cortisol, which increases the glucose in the blood and the brain’s use of glucose. However, when the demand for cortisol is consistently high, as with chronic stress, the adrenal glands can get “fatigued.” When this happens, and the NEM circuits cannot effectively handle stress, Adrenal Fatigue Syndrome (AFS) may arise.
With adrenal fatigue, stress levels, fatigue, and other unpalatable symptoms, continue to arise. This can accelerate epigenetic aging. Fortunately, proper stress management efforts can play a role in reversing epigenetic aging.
The epigenetics of exercise is one of the beneficial epigenetic influences for humans. Studies affirm that chronic and acute exercise interventions can induce different human epigenetic modifications.8 Furthermore, exercise is one of the strategies to reducing stress in the body and combating adrenal fatigue.
More research is needed on the full role of exercise on epigenetic modifications, but current reports suggest that physical exercise can have beneficial effects on DNA methylation patterns. These beneficial effects include increasing the expression of genes linked with tumor suppression. At the same time, there is a decrease in the expression of oncogenes, which are genes with the potential to cause cancer.9
The microbiome is another area of beneficial epigenetic changes for humans. A microbiome can be defined as a community of microorganisms living together in any habitat. Some organisms have colonized different parts of the body, and the gut microbiome is particularly beneficial for several reasons. For example, it helps inhibit the growth of harmful bacteria.
However, the role of the microbiome in health goes beyond that. The gut microbiome plays a role in regulating host metabolism. Alterations in the gut microbiome are associated with the pathogenesis of metabolic conditions through the induction of the three main epigenetic mechanisms.
The microbiota contributes to epigenetic regulation by influencing the availability of chemical donors for DNA methylation and histone modification, regulating epigenetic modifying enzyme expression activity, and directing epigenetic pathways by activating host-cell intrinsic processes.10
These are nutrients present in whole foods that have an epigenetic effect. Epinutrients serve to modulate the epigenetic processes that benefit us. There are two primary categories of epinutrients, and they are as follows:
 This class of epinutrients contains foods that the body uses to create methyl groups. These methyl groups are essential in epigenetic mechanisms, particularly DNA methylation. Having the right amount of these donors is important for balanced DNA methylation. Examples include folate, B12, and choline.
This class of epinutrients contains foods that the body uses to create methyl groups. These methyl groups are essential in epigenetic mechanisms, particularly DNA methylation. Having the right amount of these donors is important for balanced DNA methylation. Examples include folate, B12, and choline.
These are foods that ensure the methyl donors in the body are used in the right manner. They do not provide methyl groups for epigenetic mechanisms, but they direct DNA methylation and ensure these donors are used properly. They also help in balancing epigenetic mechanisms. Examples include curcumin and EGCG-rich foods, like apples, green tea, and avocado.
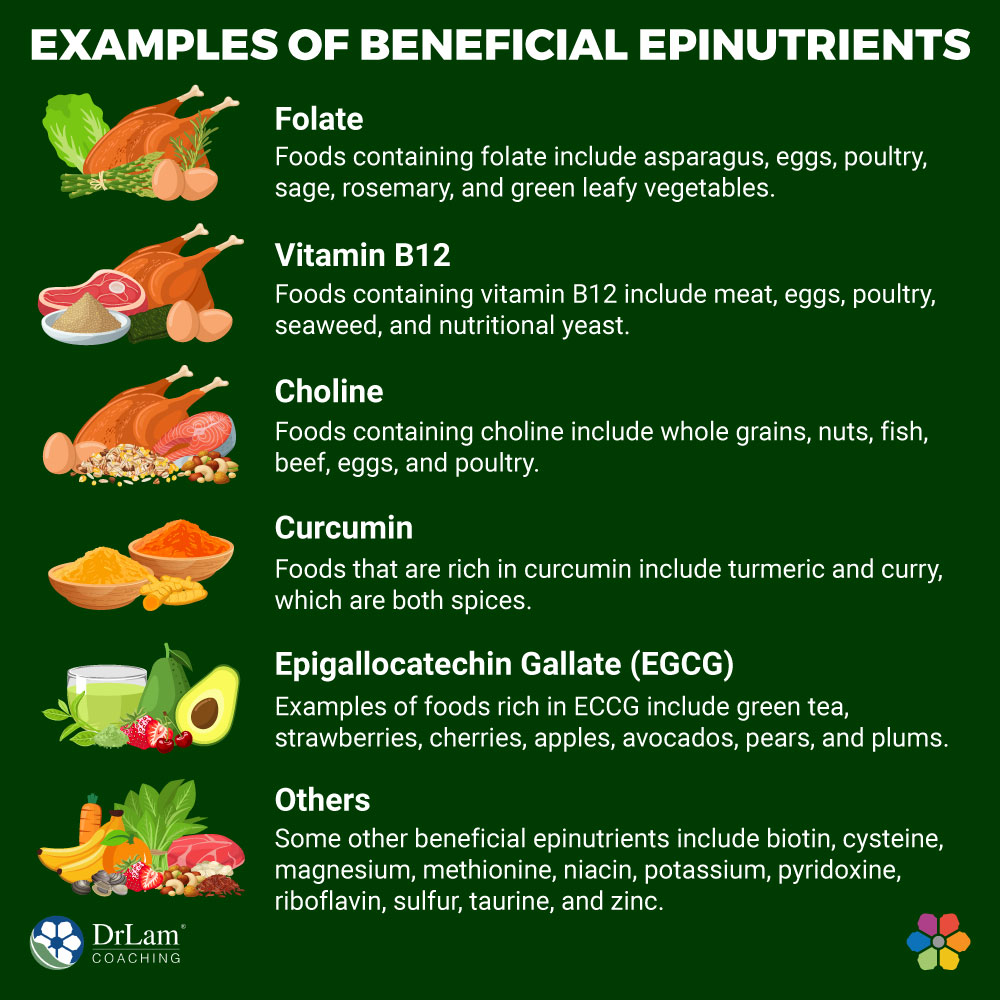
Several beneficial epinutrients fall into the two categories of epinutrients. Below are some of them:
This methyl-donor epinutrient is the most important in gene expression, DNA synthesis, and DNA repair. Also known as vitamin B9, folate donates a methyl group to homocysteine to convert it to methionine. The resultant methionine can form S-adenosylmethionine, useful in epigenetics.
Folate should not be mistaken for synthetic folic acid, which is often used to fortify foods. This is because folic acid may block active folate receptors. Also, excess folic acid in the body can have deleterious effects. Foods containing folate include asparagus, eggs, poultry, sage, rosemary, and green leafy vegetables.
This vitamin works closely with folate in mediating its beneficial methylation properties. Vitamin B12 catalyzes the reaction of homocysteine, leading to methionine. This epinutrient is also under the methyl donor class. Foods containing vitamin B12 include meat, eggs, poultry, seaweed, and nutritional yeast. If you would like a good supplement, Liponano MTHFR contains both methylcobalamin and methyl folate to help methylation and gene expression.
The link between choline and epigenetics dates back to when scientists first identified diet as useful in mediating epigenetic mechanisms. Choline is also a methyl donor but works in an indirect fashion. Foods containing choline include whole grains, nuts, fish, beef, eggs, and poultry.
This is a DNA methylation adaptogen that can help in regulating DNA methylation. It is a member of the flavonoid family and has long been used as a medicinal agent. Foods that are rich in curcumin include turmeric and curry, which are both spices. A good supplement form is Liponano Curcutrol which combines both curcumin and resveratrol to help with anti-aging.
ECCG is also a DNA methylation adaptogen and flavonoid. It can help prevent excessive DNA methylation. Also, through mild demethylation processes, ECCG can help re-express desirable genes. Examples of foods rich in ECCG include green tea, strawberries, cherries, apples, avocados, pears, and plums.
Some other beneficial epinutrients include biotin, cysteine, magnesium, methionine, niacin, potassium, pyridoxine, riboflavin, sulfur, taurine, and zinc. These are under the methyl donor class of epinutrients. For the methyl adpatogen class, other relevant epinutrients are rosmarinic acid, quercetin, and iron.
Some vitamins, like vitamins C, D, and A, fall under both categories as they have both DNA methylation adaptogen effects and active demethylating nutrients.
 Supplements are trendy in today’s modern world, chiefly because they offer the enticing promise of providing all the right nutrients and vitamins the body needs. There is just about a supplement for every condition and biological function today. However, supplements are not always advisable, especially considering they are not subject to stringent regulations by the FDA. Still, this has not deterred their use. But is supplementation advisable for gaining epigenetic benefits as opposed to just consuming epinutrients? There is no straightforward answer to that question.
Supplements are trendy in today’s modern world, chiefly because they offer the enticing promise of providing all the right nutrients and vitamins the body needs. There is just about a supplement for every condition and biological function today. However, supplements are not always advisable, especially considering they are not subject to stringent regulations by the FDA. Still, this has not deterred their use. But is supplementation advisable for gaining epigenetic benefits as opposed to just consuming epinutrients? There is no straightforward answer to that question.
With supplements, you do not get the full benefits epinutrients offer because supplements are usually concentrated to contain only particular nutrients and lack the synergistic interactions between the various components. On the other hand, some supplements can contain different low-dose nutrients working together to ensure the best possible effects.
Again, there is no assurance that supplements contain the right amount of nutrients. With epigenetic effects, the dose is as essential as the nature of the nutrients. If there are too many or too few of these nutrients, an imbalance may occur, which can disrupt the epigenome. Since the FDA does not regulate supplements with the same principles as common drugs, there is every possibility the supplement contains too much or too little of the required nutrients.
Notwithstanding, some supplements have proven helpful in providing epigenetic benefits. One such supplement is LipoNano MTHFR, which contains methylated folate and methylated vitamin B12 as its main ingredients, which both have positive epigenetic effects.
Epigenetics is the study of heritable changes in gene expression without changes to the underlying DNA sequence. Simply, it involves changes to the phenotype without changes to the genotype. Three major epigenetic mechanisms exist: DNA methylation, histone modification, and noncoding RNA gene silencing.
Epigenetic changes have real-life implications, which we can observe in sleep deprivation, exercise, stress, and the microbiome. Different environmental factors affect epigenetics, and the unique epigenetic profile of individuals determines whether the resulting influence will be positive or negative.
Epinutrients are an excellent way to gain the benefits of epigenetic changes, and they include folate, vitamin B12, curcumin, EGCG, and others. While supplements may offer some benefits, there are risks, and you should speak to your healthcare provider before starting on any new supplement.
For more information about menopause symptom management, the team at Dr. Lam Coaching can help. We offer a free, no-obligation phone consultation at +1 (626) 571-1234 where we will privately discuss your symptoms and various options. You can also send us a question through our Ask The Doctor system by clicking here.
Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009 Apr 1;23(7):781-3. doi: 10.1101/gad.1787609. PMID: 19339683; PMCID: PMC3959995.
Atlasi Y, Stunnenberg HG. The interplay of epigenetic marks during stem cell differentiation and development. Nat Rev Genet. 2017 Nov;18(11):643-658. https://pubmed.ncbi.nlm.nih.gov/28804139/
Holliday, R. Epigenetics: A Historical Overview. Epigenetics, 1:2 76-80 (2006). https://pubmed.ncbi.nlm.nih.gov/17998809/
Moosavi A, Motevalizadeh Ardekani A. Role of Epigenetics in Biology and Human Diseases. Iran Biomed J. 2016 Nov;20(5):246-58. doi: 10.22045/ibj.2016.01. Epub 2016 Jul 5. PMID: 27377127; PMCID: PMC5075137.
Kanherkar RR, Bhatia-Dey N, Csoka AB. Epigenetics across the human lifespan. Front Cell Dev Biol. 2014 Sep 9;2:49. doi: 10.3389/fcell.2014.00049. PMID: 25364756; PMCID: PMC4207041.
Gaine ME, Chatterjee S, Abel T. Sleep Deprivation and the Epigenome. Front Neural Circuits. 2018 Feb 27;12:14. doi: 10.3389/fncir.2018.00014. PMID: 29535611; PMCID: PMC5835037.
Zannas, A.S., Arloth, J., Carrillo-Roa, T. et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol 16, 266 (2015). https://doi.org/10.1186/s13059-015-0828-5
Yao B, Cheng Y, Wang Z, et al. DNA N6-methyladenine is dynamically regulated in the mouse brain following environmental stress. Nat Commun. 2017;8(1):1122 https://pubmed.ncbi.nlm.nih.gov/29066820/
Axsom JE, Libonati JR. Impact of parental exercise on epigenetic modifications inherited by offspring: A systematic review. Physiol Rep. 2019 Nov;7(22):e14287. doi: 10.14814/phy2.14287. PMID: 31758667; PMCID: PMC6874781.
Handy DE, Castro R, Loscalzo J (May 2011). “Epigenetic modifications: basic mechanisms and role in cardiovascular disease”. Circulation. 123 (19): 2145–2156. https://pubmed.ncbi.nlm.nih.gov/21576679/
Woo V, Alenghat T. Epigenetic regulation by gut microbiota. Gut Microbes. 2022 Jan-Dec;14(1):2022407. doi: 10.1080/19490976.2021.2022407. PMID: 35000562; PMCID: PMC8744890.
While there are supplements that contain nutrients that can stimulate positive epigenetic effects, the use of these supplements must be closely monitored. This is because of the lack of regulation from the FDA of these supplements, few synergistic interactions compared to epinutrients, and no assurance of correct dosing.