 The current epidemic of chronic illness not only in the U.S. but around the world has spurred considerable research into the role of normal gut flora and inflammatory markers in the development of these chronic illnesses. Much valuable information has been generated regarding inflammation as the apparent foundation of many of these conditions.
The current epidemic of chronic illness not only in the U.S. but around the world has spurred considerable research into the role of normal gut flora and inflammatory markers in the development of these chronic illnesses. Much valuable information has been generated regarding inflammation as the apparent foundation of many of these conditions.
A typical source of inflammation comes from metabolism and the lack of normal gut flora. Normal gut flora consists of the good bacteria and other microorganisms that work to digest the nutrients ingested. This process of metabolism is the body process that dictates inflammation. Metabolism is the sum total of all chemical reactions in the body that drives it toward either health or disease.
When metabolism is disrupted, inflammation results. It is this inflammation that leads to chronic illness.
Understanding the disruptors of metabolism will give healthcare professionals the information they need to develop effective strategies for dealing with chronic illnesses. There are multiple inflammatory markers that can be assessed to determine even low levels of inflammation that signals the presence of conditions ripe for the development of chronic illness.
These inflammatory markers typically result from a leaky gut barrier and/or leaky brain barrier. Both of these conditions are themselves the result of disrupted metabolism and are indicators of abnormal gut flora.
In order for a healthcare professional to determine the presence of underlying chronic inflammation, he or she must have information regarding those biochemical markers that signal that presence. Conventionally trained healthcare professionals rely mainly on the standard blood serum tests to determine the source of symptoms and illness. Serum C reactive protein (CRP) is an inflammatory marker that has historically been used as a general non-specific marker of systemic inflammation. Unfortunately, results are not very specific. Multiple conditions including acute trauma can lead to false positives.
Practitioners need to have a more comprehensive viewpoint about health and illness in order to appreciate the importance of these inflammatory markers. The NeuroEndoMetabolic (NEM) model of this stress response is that comprehensive viewpoint. This approach looks at the interaction of six organ systems in the body when stress is present. Rather than the typical approach of looking only at individual organs or symptoms, the NEM model allows practitioners to get to the root cause of symptoms. These six organ systems relate to metabolism, neuroaffective, hormonal, cardionomic, inflammatory, and detoxification processes in the body. Assessing these systems allows tracing of the cause of sometimes vague symptoms.
The vague symptoms often are related to Adrenal Fatigue Syndrome (AFS). In the normal process of responding to stress, the body ultimately stimulates the adrenal glands to secrete cortisol, the stress-fighting hormone. As stress continues, adrenal exhaustion ultimately results. At that time, the adrenals can no longer secrete cortisol. Symptoms of AFS begin. Inflammation is one of those symptoms that can start at a low level and continue there for a long time. All the time it is present, it’s causing additional problems. If stress continues, these symptoms become more serious. Ultimately, symptoms of AFS can lead a sufferer to become bed-ridden.
Assessing and dealing with the inflammatory markers of metabolic disruption allows successful remediation and a return to normal metabolism and normal gut flora.
 Much current research has been conducted that shows the importance of normal gut flora in providing energy and nutrition for the body through metabolism. The gut has been shown to be responsible for the overall health of the individual. If metabolism is disrupted, illness results.
Much current research has been conducted that shows the importance of normal gut flora in providing energy and nutrition for the body through metabolism. The gut has been shown to be responsible for the overall health of the individual. If metabolism is disrupted, illness results.
Gut flora, normal and abnormal, have been linked to a multitude of significant illnesses. Ordinarily, the thousands of microorganisms in the gut live in a symbiotic relationship that aids in metabolism.
At times of increased stress, the balance of the organisms in the gut gets interrupted, a condition called dysbiosis. This can allow food particles, viruses, and bacteria to enter the bloodstream, triggering an immune response and inflammation.
Chronic stress leads to chronic inflammation beginning in a gut with abnormal flora. Chronic inflammation leads to chronic illness conditions. Fortunately, this chronic inflammation and its relationship with disrupted metabolism can be measured using inflammatory markers.
Interleukin-6 is an vital inflammatory marker that can be tested via salivary tests. Produced at the site of inflammation, interleukin-6 (IL-6) is a major player in the acute phase of inflammation through the production of acute phase proteins. Combined with a soluble receptor sIL-6R alpha, IL-6 controls the change from acute to chronic inflammation by changing leucocyte infiltration from polymorphonuclear neutrophils to monocytes or macrophages. Chronic inflammation is also stimulated by the effects of IL-6 on T and B cells.
Acute inflammation is beneficial due to its efforts to destroy the organisms that cause infection in the body. Chronic inflammation is the kind that damages tissue and cells. Acute inflammation is predominantly made up of neutrophils initially, but this changes in a day or two to mostly monocytic cells. Chronic inflammation, on the other hand, is associated with mononuclear cells like macrophages and lymphocytes.The inflammatory marker IL-6 is involved in this change from acute to chronic inflammation, but the process is not well known.
The antibody produced for the IL-6 receptor has been used with some success in alleviating some autoimmune disorders.
Vascular Epithelial Growth Factor (VEGF) is a signal protein that enables restoration of oxygen supply to tissue when blood circulation isn’t adequate due to insufficient oxygen or other reasons. VEGF allows the body to flood specific areas with blood flow and bring the immune cells such as leukocytes or macrophages to the area much quicker than normally possible. This can lead to swelling and inflammation in the area.
When cells are not getting enough oxygen, such as in conditions of disrupted metabolism, they produce “hypoxia inducible factor” stimulating the release of VEGF. This prompts the growth of more blood vessels, bringing more oxygen to the cells. Histamine can also bring on the release of VEGF.
Another way VEGF helps immune function is by building more lymph vessels. These vessels are the way immune cells move through the body searching for bacteria, fungi, and viruses.
 Another important function of VEGF is its ability to improve neuron function and viability, thus preventing brain impairments. It can also help in preventing memory loss related to diabetes. Animal models have shown VEGF to aid in the prevention of neurodegeneration and ALS through increasing blood flow.
Another important function of VEGF is its ability to improve neuron function and viability, thus preventing brain impairments. It can also help in preventing memory loss related to diabetes. Animal models have shown VEGF to aid in the prevention of neurodegeneration and ALS through increasing blood flow.
There are some negative aspects of VEGF, as well. It can lead to increased inflammation in the body. It does this by activating microglia, which can trigger inflammation. VEGF also takes away molecules that help blood vessels stick together, thus making them more likely to leak. This can cause more inflammation and worsen the symptoms of conditions like rheumatoid arthritis.
VEGF can also increase the permeability of the blood brain barrier, increasing the likelihood of neurotoxins accessing the brain.
As VEGF builds new blood vessels, it doesn’t differentiate where it builds these vessels, sometimes increasing the blood supply to cancers. This makes it easier for cancers to grow and makes it harder to kill them.
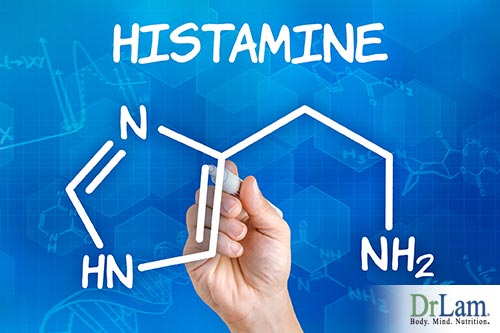 Histamine is a messenger substance in the body that aids in cellular communication. In its role as a regulator in the body, histamine works in digestion in conditions of normal gut flora, lowers blood pressure, and regulates sleep patterns. Diamine oxidase (DAO) is an important enzyme that breaks down histamine. When histamine levels are high, levels of DAO are low. DAO is found in large quantities in the cellular membranes of cells lining the small intestine and colon. People with GI problems affecting normal gut flora are much more reactive to histamine rich foods. Some medications also can inhibit DAO production and function. Leading to an increase in inflammatory markers.
Histamine is a messenger substance in the body that aids in cellular communication. In its role as a regulator in the body, histamine works in digestion in conditions of normal gut flora, lowers blood pressure, and regulates sleep patterns. Diamine oxidase (DAO) is an important enzyme that breaks down histamine. When histamine levels are high, levels of DAO are low. DAO is found in large quantities in the cellular membranes of cells lining the small intestine and colon. People with GI problems affecting normal gut flora are much more reactive to histamine rich foods. Some medications also can inhibit DAO production and function. Leading to an increase in inflammatory markers.
Because of an apparent relationship between histamine and estrogen, women are more frequently afflicted with problems related to histamine than men. With an increase in estrogen, mast cells containing histamine granulate more readily. This leads to an increase in circulating histamine and inflammation. Women who suffer from PMS or PMDD should avoid foods that are high in histamine. This is a condition that can result from AFS, as well.
Histamine in the body is produced in mast cells and basophils as a local immune response to foreign materials. It is also produced in the brain where it acts as a neurotransmitter. Another place where histamine can be stored and released is within the enterochromaffin-like cells in the stomach.
When histamine is released, it makes capillaries more permeable to white blood cells and proteins. This gives rise to the runny nose and watery eyes so familiar in allergies. When foreign antigens bind to IgE sensitized mast cells in mucous membranes, sneezing follows as a way to expel them from the body.
In the brain, the histamine neurons in the posterior hypothalamus affect wakefulness and prevent sleep. Histamines located in the stomach stimulate the parietal cells there to secrete the gastric fluids needed for digestion.
Myeloperoxidase (MPO) is an enzyme present when inflammation is present. It is released into the fluid around cells from polymorphonuclear neutrophils and macrophages. Because MPO is involved in oxidative stress and inflammation, it has been shown to be a significant marker when evaluating people with coronary heart disease.
Both oxidative stress and inflammation have been implicated in coronary problems. Macrophages and neutrophils infiltrate stable coronary artery plaques, making them unstable lesions. MPO is contained in these macrophages and neutrophils and can be measured in blood as an inflammatory marker. MPO aids in the conversion of chloride and hydrogen peroxide into hypochlorite and is secreted in inflammation. MPO reduces nitric oxide (NO) bioavailability inhibiting the ability of NO to decrease inflammation.
Along with IL-6, MPO is a good marker for the presence of inflammation.
Hypoxia inducible factor (HIF) 1 alpha is a chief regulator of the homeostatic response to hypoxia on both cellular and systemic levels. It activates genes involved in energy metabolism, angiogenesis, and apoptosis. It also activates other genes that increase oxygen delivery or increase metabolic adaptation to hypoxia.
HIF functions can also be triggered by pathological stressors such as inflammation, infections, and cancer. HIF performs functions such as increasing phagocyte microbicidal, driving T cell differentiation, and cytotoxic activity. The immune control function of HIF plays a role in cellular metabolism that is a key regulator of immunity.
HIF transcription factors play a major role in immune cell metabolism and function.
 In its role of regulator of adaptation in low-oxygen environments, HIF also regulates gene expression involved in glycolysis, erythropoiesis, angiogenesis, and proliferation. In its regulatory role in inflammation, HIF aids in the optimization of innate immunity, acts to control pro-inflammatory gene expression, helps in controlling bacterial killing, and influences cell migration.
In its role of regulator of adaptation in low-oxygen environments, HIF also regulates gene expression involved in glycolysis, erythropoiesis, angiogenesis, and proliferation. In its regulatory role in inflammation, HIF aids in the optimization of innate immunity, acts to control pro-inflammatory gene expression, helps in controlling bacterial killing, and influences cell migration.
Hypoxia and inflammation are interrelated. Inflammation in the presence of hypoxic conditions appears to be the foundation of many chronic illness conditions. Metabolic supply and demand ratios for oxygen cause stabilization of HIF-1 alpha when intestinal inflammation is present, leading to inflammatory hypoxia. These same metabolic alterations that bring on stabilization of HIF-1 alpha are associated with lung inflammation, as well. Thus, HIF-1 can be seen as an inflammatory marker
This cytokine affects growth and proliferation of many cells and has both pro- and anti-inflammatory effects. TGF-beta is produced in many cells. Some of these are macrophages, fibroblasts, microglial cells, and T and B lymphocytes.
TGF-beta 1 is the form of TGF-beta most involved in inflammation and a great inflammatory marker. People with biotoxin or mold problems will often have high levels of TGF-beta.
TGF functions as protection against autoimmune gut disorders through increasing serotonin transporters and uptake in the gut. This neurotransmitter is low in IBS and IBD.
TGF-beta also plays a role in killing T cells that invade tissue. One of the normal functions of TGF-beta is tumor suppression, although tumors can develop resistance to this substance late in their progression.
In infection conditions, TGF-beta protects against collateral damage by the immune system. Unfortunately, it also aids pathogens in evading the immune response, which leads to chronic infections.
While TGF-beta stimulates local immune and inflammatory responses, it suppresses the immune system at the systemic level. Research has shown mice who have a deficiency in TGF-beta tend to develop a significant inflammatory response that can lead to death. This suggests TGF-beta has a role in actively suppressing inflammation.
TGF can also increase inflammation. It produces pro-inflammatory Th17 and TH9 cells and inhibits TH22 in an inflammatory condition.
Another negative aspect of TGF is its ability to increase VEGF by increasing HIF, thus helping in the spread of tumors. In gastric cancer patients, a high level of TGF-beta can indicate lymph node metastasis and a poor prognosis.
TGF-beta will also make mast cells, fibroblasts, neutrophils, monocytes, and lymphocytes adhere to sites of tissue injury and inflammation. It will stimulate these cells to produce pro-inflammatory cytokines, such as IL-6, IL-1, and TNF.
It appears TGF-beta plays a role in several chronic inflammatory illness conditions.
Finally, it is important to discuss inflammation in the setting of obesity and your metabolism.
In conditions of normal gut flora and normal metabolism, the body is supplied with the energy its cells need in order to function properly. With the disruption of metabolism, this process becomes imbalanced and inflammation results.
When immune responses are triggered, inflammatory markers are sent out and inflammation begins, the metabolic process is interrupted. These changes in metabolic activity are brought on by increased recruitment of neutrophils and monocytes, both types of inflammatory cells, and by dramatic increases in lymphocyte populations. This brings a significant shift in energy supply and demand, resulting in metabolic acidosis and lowered availability of oxygen to cells leading to hypoxia significant enough to bring on serious changes in tissue metabolism.
These shifts in tissue metabolism bring on a lack of local nutrients, increased demand for oxygen, and release of an abundance of reactive nitrogen and oxygen components.
 Research into obesity and the insulin resistance that often accompanies it, along with inflammation showed obesity to be a major driving force behind immune cell infiltration which adds to metabolic inflammation. One of the major components of this metabolic inflammation is the presence of adipose tissue macrophages (ATM). The levels of these ATM increases in obese individuals anywhere from 10 to 60 percent. With increased saturated fatty acid intake, pro-inflammatory M1 genes are activated. These M1 genes contribute to insulin resistance. Typically, much of the standard American diet contains saturated fatty acids.
Research into obesity and the insulin resistance that often accompanies it, along with inflammation showed obesity to be a major driving force behind immune cell infiltration which adds to metabolic inflammation. One of the major components of this metabolic inflammation is the presence of adipose tissue macrophages (ATM). The levels of these ATM increases in obese individuals anywhere from 10 to 60 percent. With increased saturated fatty acid intake, pro-inflammatory M1 genes are activated. These M1 genes contribute to insulin resistance. Typically, much of the standard American diet contains saturated fatty acids.
The infiltration of immune cells brings inflammatory markers within metabolic tissues. These inflammatory markers can disrupt insulin signaling. Obesity also increases oxidative stress, further adding to insulin resistance.
With this information, it’s easy to see the connection between the epidemic of obesity and the epidemic of inflammation in this country. It is important to get your inflammatory markers checked.
There is likewise some significant current research indicating the effects of “meta-inflammation” in organ systems involved in metabolism. For example, the liver is involved in metabolic homeostasis, lipogenesis, gluconeogenesis, and cholesterol metabolism.
Lipotoxicity can develop from metabolic inflammation that comes about when the liver goes over its ability to store and use fatty acids. This condition is the side effect of lipid accumulation on glucose and insulin metabolism.
Lipotoxicity sets the stage for the development of potentially dangerous lipid species, impaired insulin signaling, and non-alcoholic fatty liver disease. Insulin resistance in the liver can also be caused by steatosis, which interferes with the ability of insulin to lower production of hepatic glucose and increase the synthesis of glycogen.
Lipotoxicity is an important part of metabolic inflammation.
Meta-inflammation in muscle groups is also significant. Impaired insulin signaling, such as from obesity induced metabolic inflammation, also inhibits glucose metabolism in skeletal muscles. Since muscles take care of 80 percent of glucose uptake after insulin stimulation, this process is important. Increased free fatty acids lead to lipotoxicity and decrease skeletal muscle insulin sensitivity.
The pancreas is also negatively impacted by lipotoxicity and glucotoxicity brought on by obesity. The insulin resistance seen in obesity raises the metabolic demand on the pancreas with resulting hyperglycemia when these cells can no longer keep up with their compensatory load. This hyperglycemia produces glucotoxicity that brings on a decrease in insulin gene expression in the pancreas.
It appears the proinflammatory cytokine IL-1 beta plays a major role in these disorders. A part of the process appears to be an overproduction of metabolites such as uric acid and cholesterol crystals outside the cells are signals sensed by NLRB3, which then increases the production of IL-1 beta. This line of research shows the role of metabolism during host defense and inflammation.
 With normal gut flora and a fully-functioning metabolism, the body can perform its multitude of tasks and remain healthy. But when metabolism is disrupted, inflammatory markers are released and inflammation that can ultimately lead to several serious chronic illness conditions results.
With normal gut flora and a fully-functioning metabolism, the body can perform its multitude of tasks and remain healthy. But when metabolism is disrupted, inflammatory markers are released and inflammation that can ultimately lead to several serious chronic illness conditions results.
The typical laboratory tests performed by conventionally trained healthcare professionals do not always give the type of comprehensive information needed to determine the presence of markers indicating chronic, low-level inflammation that can signal the onset or predict the likelihood of chronic illnesses. The inflammatory markers needed can be found, but are often not recognized as the markers they are.
To have a complete picture of a person’s health condition that will lead to successful remediation efforts, healthcare professionals must know about the inflammatory markers that can reveal the underlying inflammation that leads to chronic illnesses.
Even with normal gut flora, metabolism can be disrupted and develop markers to show this disruption. Some of those markers include VEGF, histamines, MPO, and TGF-beta. These markers are not always recognized by conventionally trained healthcare providers as indicators of underlying inflammation.