 Have you ever wondered what normal body ph is? Have you ever wondered if your body was unhealthy and had a normal body ph? Well, this article concerning normal body ph. is for you. Keep reading to learn about normal body ph and how it's affecting you. One of the key determinants of the speed of aging and onset of degenerative diseases is the internal biochemistry and terrain of the body. Internal biochemistry is best measured and discussed in terms of the pH. pH is a measurement of the amount of acid and base. It ranges from 1 to 14, with 1 being extremely acidic, and 14 being extremely basic. A neutral state has a pH of 7. There are many environmental factors that can affect the body's pH and it is vital for all bodily function to maintain a normal body pH. The term acidosis is and only meant to convey a shift in total body chemistry towards the acidic direction. Keep reading to learn more about normal body ph and acidosis.
Have you ever wondered what normal body ph is? Have you ever wondered if your body was unhealthy and had a normal body ph? Well, this article concerning normal body ph. is for you. Keep reading to learn about normal body ph and how it's affecting you. One of the key determinants of the speed of aging and onset of degenerative diseases is the internal biochemistry and terrain of the body. Internal biochemistry is best measured and discussed in terms of the pH. pH is a measurement of the amount of acid and base. It ranges from 1 to 14, with 1 being extremely acidic, and 14 being extremely basic. A neutral state has a pH of 7. There are many environmental factors that can affect the body's pH and it is vital for all bodily function to maintain a normal body pH. The term acidosis is and only meant to convey a shift in total body chemistry towards the acidic direction. Keep reading to learn more about normal body ph and acidosis.
According to the research of Dr. Enderlein, our bodies can only be healed of any chronic illness when our blood is at a normal, slightly alkaline pH.
What exactly does pH mean? pH is the short form for potential hydrogen. The pH of any solution is the measure of its hydrogen-ion concentration. The higher the pH reading, the more alkaline and oxygen rich the fluid is. The lower the pH reading, the more acidic and oxygen deprived the fluid is. The pH ranges from 0 to 14, with 7.0 being neutral. The indicator above 7.0 is alkaline and below 7.0 is considered acidic. So, what is normal body ph for us? keep reading to find out if you have a normal body ph.
Our blood pH has a very narrow range of around 7.35 to 7.45. If our body's pH deviates from this range, we will be sick or have symptoms of falling sick. If our blood pH falls below 6.8 or above 7.8, our body cells will stop functioning and death will occur.
A normal healthy body will have an almost equal blood pH of acidity and alkalinity. An ideal pH balance is 7.4, which means that it is slightly more alkaline than acid. Only when the pH level is balanced that our bodies can then effectively assimilate vitamins, minerals and food supplements. As such, our body's pH determines everything. So, with a normal body ph, we can essentially be healthier.
A body with an acidic pH is more prone to illness. In an acidic environment, red blood cells cannot repel and stick together like a stack of coins, form what is called rouleaux formation. This formation limits the amount of oxygen carrying capacity because red blood cells sandwiched between the two ends are compressed against each other and therefore unable to carry oxygen. Reduced oxygen leads to fatigue, lack of energy, and weakness, just to mention a few symptoms. Furthermore, cancer cells strive in an oxygen deprived environment (anaerobic) much better than in an oxygen rich environment.
The importance of maintaining a normal body pH is, therefore, a critical factor in balancing proper internal terrain to deter cancer, infection, and a host of inflammatory disease. The majority of these conditions worsen in an acidic environment. They do not do well in an acidic environment and a normal body ph. isnt highly acidic.
The normal human cell is slightly alkaline and has an abundance of molecular oxygen. The cancer cell is acidic and cannot survive in an oxygen-rich environment. As such, we can conclude that pH balance is very important to one's health, especially for the cancer patient.
 The pH indicators are an exponent number of 10. A small difference in pH will translate to a big difference in the number of oxygen or OH-ions. A difference of 1 in a pH value means ten times the difference in the number of OH-ions. A difference of 2 means one hundred times the difference in the number of OH-ions. In other words, a blood with a pH value of 7.45 contains 64.9% more oxygen than blood with a pH value of 7.30.
The pH indicators are an exponent number of 10. A small difference in pH will translate to a big difference in the number of oxygen or OH-ions. A difference of 1 in a pH value means ten times the difference in the number of OH-ions. A difference of 2 means one hundred times the difference in the number of OH-ions. In other words, a blood with a pH value of 7.45 contains 64.9% more oxygen than blood with a pH value of 7.30.
Acidosis is a pH imbalance where the body has accumulated too much acid and does not have sufficient acid neutralizer to neutralize the effects. Acidosis may result from a lack of insulin, a starvation diet or even a gastrointestinal disorder like vomiting and diarrhea.
The acidic biochemical type is not meant to represent the absolute pH measurement of the blood system, which generally requires a laboratory test performed in hospitals known as blood gasses. While certain areas of the body normally may have a more acidic environment (stomach, kidneys, bladder) than others, the overall pH of the body and blood normally must be maintained within a narrow range of alkalinity (pH 7.30 - 7.45). An absolute blood measurement of acidity (pH below 7.0) is incompatible with life.
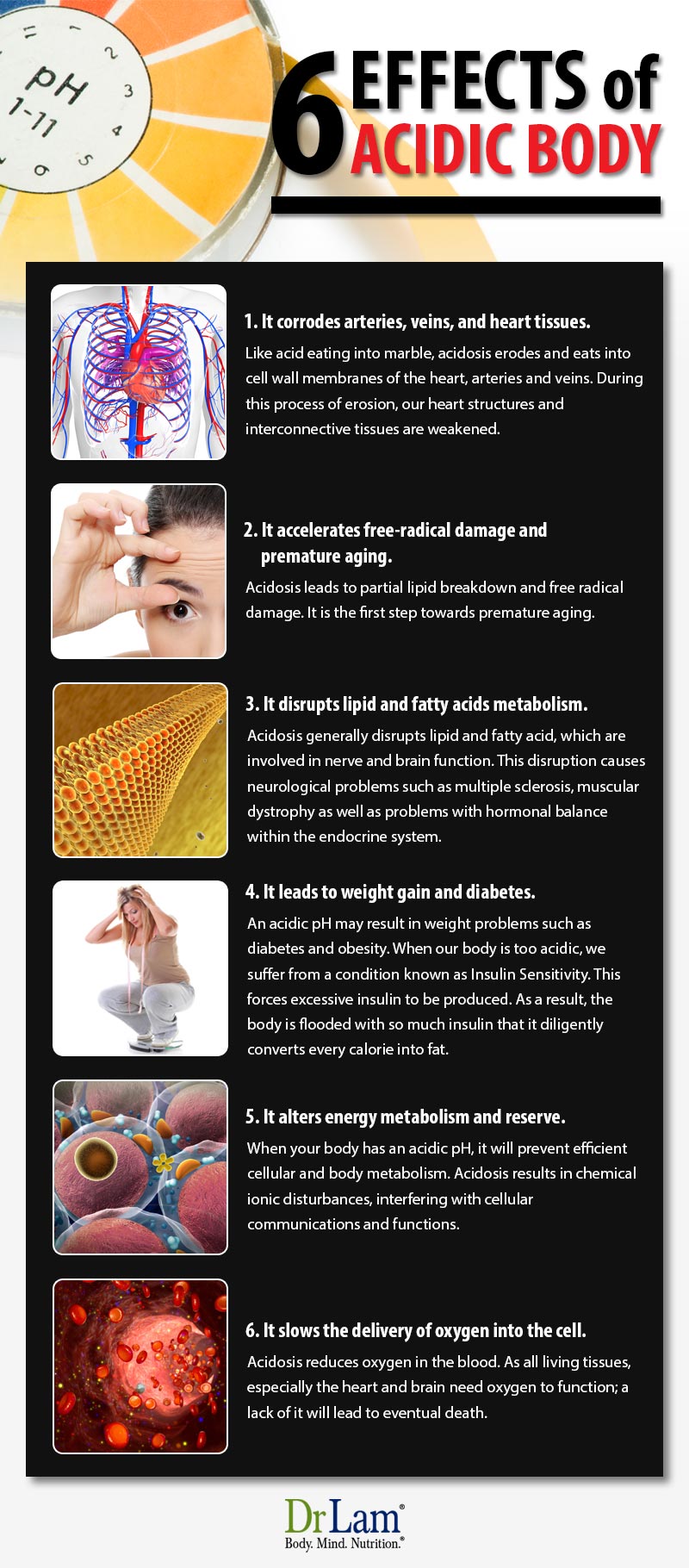
Acidosis leads to serious problems with major organs such as the liver, heart or kidneys. In this article, we will be looking into some of the reasons as to why we should avoid acidosis.
Acid pH changes of blood are now thought to result in the following:
The structural weakening of the cardiocascularity also creates irregularities of blood pressure, which further exacerbates the above problems.
 An acidic pH may result in weight problems such as diabetes and obesity. When our body is too acidic, we suffer from a condition known as Insulin Sensitivity. This forces excessive insulin to be produced. As a result, the body is flooded with so much insulin that it diligently converts every calorie into fat.It is very likely that an acid pH, from an imbalanced diet, produces a condition, which stimulates the predetermined genetic response to starvation and famine. Thereafter, the body will have to increasingly hoard every calorie consumed and store it as fat.Some people reckon that an acid pH immediately signals the powerful genetic response to an impending famine, directly interpreting with the all important and very sensitive Insulin-Glucagon Axis. When this happens, it makes the body produce more insulin than usual, and in turn, produce more fats and store it.
An acidic pH may result in weight problems such as diabetes and obesity. When our body is too acidic, we suffer from a condition known as Insulin Sensitivity. This forces excessive insulin to be produced. As a result, the body is flooded with so much insulin that it diligently converts every calorie into fat.It is very likely that an acid pH, from an imbalanced diet, produces a condition, which stimulates the predetermined genetic response to starvation and famine. Thereafter, the body will have to increasingly hoard every calorie consumed and store it as fat.Some people reckon that an acid pH immediately signals the powerful genetic response to an impending famine, directly interpreting with the all important and very sensitive Insulin-Glucagon Axis. When this happens, it makes the body produce more insulin than usual, and in turn, produce more fats and store it.
On the other hand, a healthy and slightly alkaline pH will yield normal fat burning metabolic activities, making no demands on the body to produce extra insulin and make fats. As such, this allows fat to be burned and naturally lost. A healthy pH diet is also less likely to have any yo-yo effects, or rebounding from a diet with additional weight gain. We should try to maintain a healthy alkaline pH so as to allow fats to be burnt normally for energy, rather than hoarded and stored under the mistaken biochemical belief of an impending famine.
Acidosis also disrupts the insulin-producing pancreatic beta cells. These beta cells are especially sensitive to pH and cannot survive if the body is too acidic. When this occurs, beta cells will lose phase with one another. Their cellular communication will be thwarted and the body's immune system will start to over-respond. Stress within the cells will increase, making them more difficult to perform adequately and survive.
It is important to note that the body's biochemistry is an important but just one of many tools to help the physician understand the whole body. Not having a normal body pH in and of itself is not a diagnostic tool and is not a medical diagnosis as a disease entity.
What happens when the body is too acidic? A body pH that is too acidic will:
 Some people who have high acidity levels tend to exhibit these symptoms such as: anxiety, diarrhea, dilated pupils, extroverted behavior, fatigue in early morning, headaches, hyperactivity, hyper sexuality, insomnia, nervousness, rapid heartbeat, restless legs, shortness of breath, strong appetite, high blood pressure, warm dry hands and feet.
Some people who have high acidity levels tend to exhibit these symptoms such as: anxiety, diarrhea, dilated pupils, extroverted behavior, fatigue in early morning, headaches, hyperactivity, hyper sexuality, insomnia, nervousness, rapid heartbeat, restless legs, shortness of breath, strong appetite, high blood pressure, warm dry hands and feet.
Most of the time, the body becomes acidic due to a diet rich in acids, emotional stress, toxic overload, and/or immune reactions or any process that deprives the cells of oxygen and other nutrients. When this happens, the body will try to compensate for acidic pH by using alkaline minerals such as calcium. As a result, calcium is removed from the bones, causing osteoporosis.
Acidosis, which is an extended time in the acid pH state, can result in rheumatoid arthritis, diabetes, lupus, tuberculosis, osteoporosis, high blood pressure and most cancers.
Two main factors leading to cancer are an acidic pH and a lack of oxygen. As such, are we able to manipulate these two factors so as to prevent and control cancer?
Everyone knows that cancer needs an acidic and low oxygen environment to survive and flourish. Research has proven that terminal cancer patients have an acidity level of 1,000 times more than normal healthy people. The vast majority of terminal cancer patients have a very acidic pH. Why is this so?
The reason is simple. Without oxygen, glucose undergoing fermentation becomes lactic acid. This causes the pH of the cell to drop to 7.0. In more advanced cancer cases, the pH level falls further to 6.5. Sometimes, the level can even fall to 6.0 and 5.7 or lower. The basic truth is that our bodies simply cannot fight diseases if our pH is not properly balanced.
The body undergoes a natural and ongoing balancing act constantly. Underlying regulatory forces work continually to balance an acidic body chemistry to remove excess acid and return the body to a more neutral state.
These internal buffering mechanisms include:
 a. Calcium Imbalance. Many minerals and other factors involved in maintaining a normal pH fluctuate throughout the day in response to variations in serum and electrolyte concentrations, as well as dietary influences. Such minerals act as a buffer and a balancer to maintain the body in a balanced chemistry state in order for proper enzyme activations to occur. Long term acidosis can lead to mineral depletion and osteoporosis. Calcium, for example, has been shown to enter the intracellular compartment in an effort to maintain ionic balance when magnesium is deficient, resulting in calcification of the cell and resulting hypertension. High dose of calcium supplementation has been shown to reduce hypertension.
a. Calcium Imbalance. Many minerals and other factors involved in maintaining a normal pH fluctuate throughout the day in response to variations in serum and electrolyte concentrations, as well as dietary influences. Such minerals act as a buffer and a balancer to maintain the body in a balanced chemistry state in order for proper enzyme activations to occur. Long term acidosis can lead to mineral depletion and osteoporosis. Calcium, for example, has been shown to enter the intracellular compartment in an effort to maintain ionic balance when magnesium is deficient, resulting in calcification of the cell and resulting hypertension. High dose of calcium supplementation has been shown to reduce hypertension.
b. High Uric Acid levels, resulting from the breakdown of meat byproducts (purines), contribute to the development of an acidotic condition. Excessive protein intake, including meats, viscera, and leguminous vegetables increases uric acid production. Other causes of hyperuricemia include gout, renal failure, lactic acid buildup, alcohol abuse, extreme exercise and pregnancy, malignant neoplasms, and the use of diuretics.
c. High Triglyceride levels, resulting from the breakdown of endogenous fat or excessive ingestion of sugar (including grains).Diets excessive in complex carbohydrates (such as pasta, bread, and starches) is the most common cause of elevated triglyceride levels. Only 20% of the ingested sugar load can be burned or stored as glycogen at any one meal. The remainder 80% will be converted to triglyceride which can contribute to the buildup of acidity, or stored as fat deposits.
Just wet a piece of litmus paper with your saliva 2 hours after a meal and this will give you a reflection of your state of health.
Although saliva is generally more acidic than blood, it is a fairly good indicator of health. It tells you what your body retains. Salivary pH is a fair indicator of health for extracellular fluids and their alkaline mineral reserves.
The optimal pH for saliva is between 6.4 to 6.8. A reading lower than 6.4 means that there are not enough alkaline reserves. After meals, the saliva pH should rise to 7.8 or higher. If there is no increase, it will imply that the body has a deficiency in alkaline minerals especially calcium and magnesium. Food will not be absorbed and assimilated well. To deviate from an ideal salivary pH for an extended time can lead to illnesses.
If the salivary pH level remains too low, we should take more fruits, vegetables and mineral water and avoid strong acidifiers such as sodas, whole wheat and red meat to maintain its balance.
The pH of the urine is an indication as to how well the body is working to maintain a proper pH of the blood. It reflects the efforts of the body via the kidneys, adrenals, lungs and gonads through the buffer salts and hormones. The urine also shows the alkaline building (anabolic) and acid tearing down (catabolic) cycles. By taking urine samples, we can have access to a fairly accurate picture of our body chemistry as our kidneys filter out the buffer salts of pH regulation and provide values based on what the body is eliminating. The urine pH can vary from around 4.5 to 9.0, but the ideal range is still 5.8 to 6.8.
To increase the alkalinity in our blood and encourage a normal body ph, we can consume these foods: almonds, aloe vera, apples, apricots, bee pollen, buckwheat, cabbage, cantaloupe, celery, carrots, cucumbers, dairy products except hard cheese, dates, pulse, poached eggs, figs, grapefruit, honey, lettuce, millet, parsley, raisins, peaches, fresh red potatoes, pineapple, soy products, sprouted seeds, cooked spinach, turnip tops, wakame miso soup, azuki beans, rice and mineral water.
In conclusion, balancing your pH is a major step towards optimal well-being and better health. Acidosis is the main cause of calcium deficiency disease. After many studies, scientists can safely conclude that healthy people have body fluids that are alkaline (high pH) whereas sick people have body fluids that are acidic (low pH).
The blood is normally maintained in an alkaline state. An acidic state reduces blood oxygen carrying capacity and promotes a higher valence state of polymorphism.
Foods are generally broken down and converted into either acidic or alkaline substrate with digestion. A person with an acid metabolic condition should emphasize the consumption of alkaline forming foods in the diet, with 60-70% alkaline forming foods, and 30-40% acid forming food by volume.
Do not simply eat all alkaline forming food to sustain normal body ph, as some acid formation is necessary to generate metabolic activity and maintain homeostasis. Too alkaline a body can predispose the body to certain types of infection.
Here are some of the common acid and alkaline forming foods. These categories are based on the body's reaction to a moderate amount of each. Excess amounts may produce the opposite effect. A general rule of thumb, vegetables, and fruits are alkaline and high protein food and high sugar foods are acidic. They encourage balance and a normal body ph.
Dairy, Eggs, Meat, Poultry, Fish, Grains, Processed flour products, Sugary foods, Cranberry, Pomegranate, Plum, Prune, Tomato, most Legumes, most Grains. These foods can discourage normal body ph.
Kelp, Sea salt, Seaweeds, Parsley, Onion, Miso, Daikon, Sea Vegetables, Burdock root, lotus root, Sweet Potato/Yam, Nectarine, Persimmon, Raspberry, Watermelon, Tangerine, Pineapple
© Copyright 2004 Michael Lam, M.D. All Rights Reserved.