 Human microbiome is defined as the entire habitat, including the microorganisms, their genomes (i.e., genes) and their surrounding environmental conditions. The word itself is derived from the word biome which refers to the characteristics of an environment including abiotic (nonliving) as well is biotic (living) factors. Human microbiome can easily be mistaken from another commonly encountered term called microbiota, which is the composition of the microorganism population within a defined environment, such as the GI tract or oral cavity. An estimated 90 percent of cells found in the human body are not human after all but of mostly prokaryotic origin, derived from at least 40,000 bacterial strains in 1,800 genera. Though considerably smaller in size, these approximately 100 trillion cells weigh up to five pounds in an adult individual – approximately the weight of a full-grown human brain. They inhabit in all parts of the body that are exposed to the environment, such as the mouth, skin, and vagina. The biggest population resides in the gut where healthy gut bacteria have a constant supply of the nutrients necessary for their survival. Taken collectively, these organisms outnumber our human cells 10:1.
Human microbiome is defined as the entire habitat, including the microorganisms, their genomes (i.e., genes) and their surrounding environmental conditions. The word itself is derived from the word biome which refers to the characteristics of an environment including abiotic (nonliving) as well is biotic (living) factors. Human microbiome can easily be mistaken from another commonly encountered term called microbiota, which is the composition of the microorganism population within a defined environment, such as the GI tract or oral cavity. An estimated 90 percent of cells found in the human body are not human after all but of mostly prokaryotic origin, derived from at least 40,000 bacterial strains in 1,800 genera. Though considerably smaller in size, these approximately 100 trillion cells weigh up to five pounds in an adult individual – approximately the weight of a full-grown human brain. They inhabit in all parts of the body that are exposed to the environment, such as the mouth, skin, and vagina. The biggest population resides in the gut where healthy gut bacteria have a constant supply of the nutrients necessary for their survival. Taken collectively, these organisms outnumber our human cells 10:1.
The fetal gut is sterile and is established at birth with microbes from the mother’s vaginal and fecal microbiome as well as with other environmental microbes confronted in the first days of life. Early colonization depends on the delivery mode, diet (breastfeeding vs. formula feeding), hygiene, and antibiotic treatment. The first colonizers are facultative anaerobes (e.g., Escherichia coli and Streptococcus spp.) and obligate anaerobic species, which colonize as oxygen levels in the gut decrease. A child’s microbiome stabilizes and becomes adult-like at three years of age. Throughout adulthood, our human microbiome is in a continuous process of self-balancing to help the body maintain homeostasis.
As we grow into old age, changes in our human microbiome occur, resulting in decreased microbial diversity, and with that, increased inflammation.
Most of the microbes in our body are not harmful at all, but help in maintaining processes that are necessary for a healthy body. They do not cause diseases under normal circumstances, and are deemed to be members of the normal flora or normal human microbiome. Our body needs healthy gut bacteria for a multitude of functions including the carrying out of various enzymatic reactions that otherwise we cannot do on our own, or synthesis of vitamins such as vitamin K necessary for good health.
 Metabolism is a term used to describe all chemical reactions involved in maintaining the living state of the cells and the organism. It is closely linked to nutrition and the availability of nutrients. Healthy gut bacteria are responsible for breaking down the complex molecules found in some foods such as vegetables and meats. They therefore assist in our metabolism. These microbes are not only responsible for harvesting energy for themselves from the plant fibers we consume, but also breaking down such plants into smaller molecules so our body can digest them easily. A simple study in mice showed that certain bacteria are associated with metabolic derangement such as obesity. When obese mice were injected with the gut microbiome of normal mice, the obese mice lost weight completely. The reverse was also true, i.e., when normal mice were injected with the microbiome of obese mice, the normal mice gained weight. Similar studies using human twins, different in weight, with similar upbringings and identical genomes, manifested the same association between obesity and human microbiome from the gut.
Metabolism is a term used to describe all chemical reactions involved in maintaining the living state of the cells and the organism. It is closely linked to nutrition and the availability of nutrients. Healthy gut bacteria are responsible for breaking down the complex molecules found in some foods such as vegetables and meats. They therefore assist in our metabolism. These microbes are not only responsible for harvesting energy for themselves from the plant fibers we consume, but also breaking down such plants into smaller molecules so our body can digest them easily. A simple study in mice showed that certain bacteria are associated with metabolic derangement such as obesity. When obese mice were injected with the gut microbiome of normal mice, the obese mice lost weight completely. The reverse was also true, i.e., when normal mice were injected with the microbiome of obese mice, the normal mice gained weight. Similar studies using human twins, different in weight, with similar upbringings and identical genomes, manifested the same association between obesity and human microbiome from the gut.
Research has also shown a direct relationship between diet and the thriving of certain gut microbial communities. This should be rather obvious. For instance, vegetarians have gut flora that is better equipped for breaking down plant roughage, making the otherwise indigestible molecules, such as cellulose, available for humans. During the bacterial metabolism of the complex molecules, chemical signals that are released end up in our brains and can affect behavior. It is not a great speculative leap to then consider that the microbes responsible for these chemical signals may be behind certain food cravings and have an effect on what we choose to eat. Clearly, the association between our human microbiome, healthy gut bacteria and metabolism is far greater than we envisioned.
It is interesting to note that at specific sites on the body, entirely different sets of microbes could perform identical functions for different people. For example, completely separate families of microorganisms existing on the tongues of different individuals will perform the same breakdown of sugars. So what are the functions of all of the microbes living in our bodies? The microbes benefit from a persistent, stable habitat, rich in energy from the food we ingest, and we in turn claim heat energy from the bacterial breakdown of compounds like cellulose that are indigestible by the human gut. The interaction between the human body and the microbes it plays host to is far reaching. The internal functions that go on in the body, which are essential for a healthy body, are dependent on specific microbiomes at various sites throughout the body. When human microbiome becomes imbalanced or altered, it is known as dysbiosis.
Dysbiosis can lead to systemic inflammation, which is associated with a wide array of diseases and conditions including heart disease, diabetes, obesity, asthma, and even autism. Dysbiosis is also being implicated in several inflammatory bowel related problems, notably those that are symptom-based such as ulcerative colitis and Crohn’s disease. There is also a strong association with the brain, triggering irritable bowel syndrome (IBS). Each of these debilitating conditions has strong links to shifts in healthy gut bacteria.
A common cause of dysbiosis is ingestion of antibiotics that destroy symbiotic and competing strains necessary for optimum balance. When our normal human microbiome is altered, our immune system can become negatively affected.
 Early in life, human microbiome from the gut plays an integral role in the formation of a very strong immune system in humans, especially during our early childhood as the adaptive immune system develops. During this time, our immune system becomes accustomed to the foreign antigens in our body and develops a tolerance for such foreign antigen. Once a homeostasis is built, non-pathogenic microbes and the other harmless antigens will not provoke an inflammatory response. This is a good adaptive response to keep our body in a steady state. Over exposure to allergens, when combined with a weakened or underdeveloped immune system, can trigger inflammatory responses that are pathological, such as acute allergies, food sensitivities, chemical sensitivities, and autoimmune diseases. This concept is principally illustrated in germ-free mice (i.e., mice kept sterile throughout their life). These sterile mice are exceptionally unhealthy and have drastically underdeveloped immune systems. They exhibit undesirable traits and suffer from autoimmune diseases.
Early in life, human microbiome from the gut plays an integral role in the formation of a very strong immune system in humans, especially during our early childhood as the adaptive immune system develops. During this time, our immune system becomes accustomed to the foreign antigens in our body and develops a tolerance for such foreign antigen. Once a homeostasis is built, non-pathogenic microbes and the other harmless antigens will not provoke an inflammatory response. This is a good adaptive response to keep our body in a steady state. Over exposure to allergens, when combined with a weakened or underdeveloped immune system, can trigger inflammatory responses that are pathological, such as acute allergies, food sensitivities, chemical sensitivities, and autoimmune diseases. This concept is principally illustrated in germ-free mice (i.e., mice kept sterile throughout their life). These sterile mice are exceptionally unhealthy and have drastically underdeveloped immune systems. They exhibit undesirable traits and suffer from autoimmune diseases.
It is clear that the human microbiome that is first developed in the gut of an infant, can have a perpetual effect on his or her health. Scientists have compared babies delivered through C-section, where the newborn is colonizing its mother’s skin biome, and babies vaginally delivered, where the infant is colonizing its mother’s vaginal and gut biomes. Babies delivered through C-section have a greater likelihood of developing obesity and allergies than their vaginally delivered counterparts.
 When dysbiosis occurs at the GI tract, resulting inflammation can lead to damaged mucosa. The tight junction between mucosal cells is compromised and triggers further immune system responses. Unwanted proteins, toxins, and bacteria can more easily pass through the damaged lining of the gut and enter our systemic circulation. This is often referred to as a leaky gut. This can result in brain fog, depression, pain of unknown origin, fatigue, anxiety, insomnia, just to mention a few.
When dysbiosis occurs at the GI tract, resulting inflammation can lead to damaged mucosa. The tight junction between mucosal cells is compromised and triggers further immune system responses. Unwanted proteins, toxins, and bacteria can more easily pass through the damaged lining of the gut and enter our systemic circulation. This is often referred to as a leaky gut. This can result in brain fog, depression, pain of unknown origin, fatigue, anxiety, insomnia, just to mention a few.
On the other hand, having healthy gut bacteria and diverse human microbiome in the gut, and therefore no gut inflammation and leakiness, supports a person’s immune system. Those with healthy human microbiome in the gut tend to be physically strong and less prone to infections and recurrent illnesses. Their defense system is simply stronger.
The human microbiome in the intestinal track hasa strong connection to the brain. A bi-directional communication channel connecting the gastrointestinal and neural systems, also known as the gut-brain axis, coordinates the behavior of healthy gut bacteria with brain activity. This axis has a significant impact on brain development and behavior. It is evidenced that this extended communication system affects a broad spectrum of diseases, including psychiatric disorders, irritable bowel syndrome, and demyelinating conditions such as multiple sclerosis.
The brain can directly affect human microbiome in the gut, via signaling molecules discharged into the gut lumen from the cells in the enterochromaffin cells, neurons, and immune cells. The brain can also influence the enteric human microbiome directly or indirectly, via changes in gastrointestinal secretion, motility, and permeability of the intestine.
The reverse is also true in this bi-directional gut-brain highway. Communication from human microbiome ni the gut to the brain can occur via epithelial-cell, receptor-mediated signaling, and – when the intestinal permeability is improved – through direct stimulation of the immune cells. The disruption of these bi-directional interactions between human microbiome in the gut and the nervous system may be involved in the pathophysiology of chronic and acute gastrointestinal disease states, including the functional and inflammatory bowel disorders.
 The gut is therefore, called our “second brain” for good reasons. In addition, many neurotransmitters, including serotonin, are made in the GI tract. Microbes normally present in the gut stimulate host intestinal cells to produce up to 90 percent of the body’s serotonin. Peripheral serotonin is produced in the digestive tract by enterochromaffin (EC) cells and also by particular types of immune cells and neurons. Microbes are needed by the EC cells to make serotonin. EC cells are therefore rich sources of serotonin in the gut. Without proper healthy gut bacteria, serotonin, also called our “feel-good neurotransmitter” is severely compromised. Changes in levels of peripheral serotonin have been linked to conditions such as IBS, cardiovascular disease, depression, and osteoporosis.
The gut is therefore, called our “second brain” for good reasons. In addition, many neurotransmitters, including serotonin, are made in the GI tract. Microbes normally present in the gut stimulate host intestinal cells to produce up to 90 percent of the body’s serotonin. Peripheral serotonin is produced in the digestive tract by enterochromaffin (EC) cells and also by particular types of immune cells and neurons. Microbes are needed by the EC cells to make serotonin. EC cells are therefore rich sources of serotonin in the gut. Without proper healthy gut bacteria, serotonin, also called our “feel-good neurotransmitter” is severely compromised. Changes in levels of peripheral serotonin have been linked to conditions such as IBS, cardiovascular disease, depression, and osteoporosis.
Human microbiome also has an important influence on the behavior of its host. Many nerve endings are positioned around the gut, which transmits signals straight to the brain via the vagus nerve. Metabolites and other minute molecules that are released from healthy gut bacteria can influence everything from taste to mood. In fact, in a study, scientists swapped the microbiome of risk-taking mice with that of cowardly mice and their risk-aversion interchanged as well. Other studies have hypothesized that the types of food we crave, as well as what tastes good to us, can also be commanded by the human microbiome population in our guts. It may even be related to the human microbiome population's ability to make use of particular foods for energy.
The liver is the body’s major metabolic clearing house. It is responsible for breaking down food, toxins, nutrients, medications, etc into smaller component parts called metabolites and prepare them for excretion as part of the body’s overall detoxification process to keep us clean internally. The interaction between the innate immune system and the intestinal microbiota during obesity or autoimmunity promotes chronic liver disease progression. There is a central relationship between the immune system, human microbiome, and liver disease initiation and progression.
Liver disease has long been associated with qualitative and quantitative (overgrowth) dysbiotic changes in normally healthy gut bacteria of the intestinal microbiota. Unhealthy external factors, such as high sugar, a fatty Western diet, and alcohol, can alter the homeostasis. Dysbiosis results in intestinal inflammation, a breakdown of the intestinal barrier, and translocation of microbial toxic products across the intestinal wall into the liver, which can promote liver injury and inflammation. It is clear that human microbiome–immunologic–liver interaction plays an important role in overall health.
 The association between adrenal fatigue and microbiome is more than casual. The microbiome is defined as the entire habitat, including the microorganisms, their genomes (i.e., genes) and their surrounding environmental conditions. Although dysbiosis – a disruption in our microbiome when healthy gut bacteria become imbalanced or altered – is one of the most frequent aggravating factors of issues with the microbiome and Adrenal Fatigue, it is often overlooked.
The association between adrenal fatigue and microbiome is more than casual. The microbiome is defined as the entire habitat, including the microorganisms, their genomes (i.e., genes) and their surrounding environmental conditions. Although dysbiosis – a disruption in our microbiome when healthy gut bacteria become imbalanced or altered – is one of the most frequent aggravating factors of issues with the microbiome and Adrenal Fatigue, it is often overlooked.
Adrenal fatigue is a condition describing a weakened adrenal state of function due to stress intolerance. The primary symptoms are fatigue and lack of energy. It represents the neuroendocrine stress response by the body, as it tries to overcome stress by way of the hypothalamic-pituitary-adrenal (HPA) hormonal axis. Routine laboratory tests are usually normal, and sufferers are normally sent home and told nothing is wrong by conventionally trained doctors after Addison’s Disease has been ruled out.
There are four stages of adrenal fatigue. In early stages (stage 1 and 2), symptoms are very mild and subtle, and are often passed over, with complaints such as sugar craving, afternoon slump, and exercise intolerance compensated by snacking, napping, and reduced physical activity respectively. Advanced stages of adrenal fatigue (stage 3 and 4) are much more serious and can be incapacitating, with symptoms including reactive hypoglycemia, insomnia, low blood pressure, salt craving, dizziness, heart palpitations, menstrual irregularity, depression, panic attacks, and weight loss. Some are even bedridden requiring ambulatory help.
It is well known that gut pathogens such as Escherichia coli, if they enter the gut, can activate the HPA. However, animals raised in a germ-free environment show exaggerated HPA responses to psychological stress as well. Stress induces increased permeability of the gut, especially at the tight junction, which is the gap between mucosal cells that is usually hard to permeate. As the tight junction becomes more lax, toxins, bacteria, and bacterial antigens cross the epithelial barrier and enter systemic circulation, bringing cellular signals to the brain distally. Locally, mucosal immune response is activated, which in turn alters the composition of the microbiome and leads to enhanced HPA drive. It is clear that healthy gut bacteria must be taken into account when considering these factors that regulate the HPA both locally and systemically.
Research is currently exploring the interactions between gut microbiome and adrenal fatigue syndrome. Our healthy gut bacteria is therefore, directly connected to how well our brain handles stress, and ultimately, adrenal gland function. GI symptoms directly associated with advanced adrenal fatigue include bloating, constipation, food intolerance, gastric discomfort, diarrhea, intolerance to medications and supplements, and paradoxical reactions, just to mention a few. These symptoms usually improve as healthy gut bacteria is optimized. Studies into microbiome and adrenal fatigue may offer new insights into prevention and treatment possibilities.
 Multiple pathways are involved on how dysbiosis can affect healthy gut bacteria. They include systemic GI assimilation slowdown as the body tries to conserve energy, metabolic by-products build up due to liver sluggishness, extracellular matrix congestion, and receptor site damage. Dysbiosis is an unavoidable part of the big picture as normal healthy gut bacteria fails to normalize the body in its attempt to retain homeostasis when dysbiosis is rampant. Excessive inflammation appears to be a major common final pathway pathophysiologically with dysbiosis.
Multiple pathways are involved on how dysbiosis can affect healthy gut bacteria. They include systemic GI assimilation slowdown as the body tries to conserve energy, metabolic by-products build up due to liver sluggishness, extracellular matrix congestion, and receptor site damage. Dysbiosis is an unavoidable part of the big picture as normal healthy gut bacteria fails to normalize the body in its attempt to retain homeostasis when dysbiosis is rampant. Excessive inflammation appears to be a major common final pathway pathophysiologically with dysbiosis.
When faced with excessive stress, our body microbiome changes. Studies in squirrels living in a low-stress environment have shown that they harbor healthier communities of microorganisms. In the same squirrels examined two weeks later, studies have found that if stress levels increased, some bacteria that are potentially harmful also increased. The greater the stress in the squirrels, the less bacterial diversity are found, which can be an indicator of poor health.
Bad bacteria overgrowth and resulting GI dysbiosis are prominent clinical challenges as adrenal fatigue worsens to the advanced stages. It can overwhelm and reduce the good bacteria load. When the fungi and bad bacteria overwhelm our intestines, they can stimulate inflammation, which has the direct effect of damaging the gut track locally. If not resolved, systemic inflammation is part of the natural progression as adrenal fatigue worsens. The more bad bacteria are present, the more burden is placed on the adrenal glands to produce more anti-inflammatory hormone cortisol. As further disruption of healthy microbiome and adrenal fatigue symptoms worsen, the body weakens. Eventually, the adrenal glands become exhausted. Cortisol output starts to fall after reaching a peak. As cortisol level falls, inflammation increases. Inflammation is a silent killer. It damages the body even more, thus requiring more cortisol to help repair it. The adrenals are put to work even harder. This forms a vicious cycle in which the body eventually crashes as it runs out of steam. Facing a threat to its survival, the body starts an automatic shut down process of non-essential functions to conserve energy. This is also destabilizing. Without the nutrition reserves or replenishment to be sufficient for what is needed to fight stress, the body enters a state of negative nutritional reserve, creating a vicious cycle of ever lowering energy and ultimately a catabolic state as the body surrenders and assumes a low energy vegetative state as a survival method of last resort. Sufferers become bedridden, with slow GI function, and rampant systemic inflammation driven in part by severe dysbiosis.
The gut microbiome therefore plays a central role in adrenal fatigue. Restoring healthy a composition of gut microbiome and adrenal fatigue treatment should be seen as one in the same. Modulating the gut microbiome should be considered an integral therapeutic technique to adrenal fatigue recovery. Failure to restore a normal microbiome and adrenal fatigue may worsen. This can lead to retarded recovery, frequent relapse, lower threshold to adrenal crashes, frequent infections, the pain of unknown origin, metabolic derangements, brain fog, and depression.
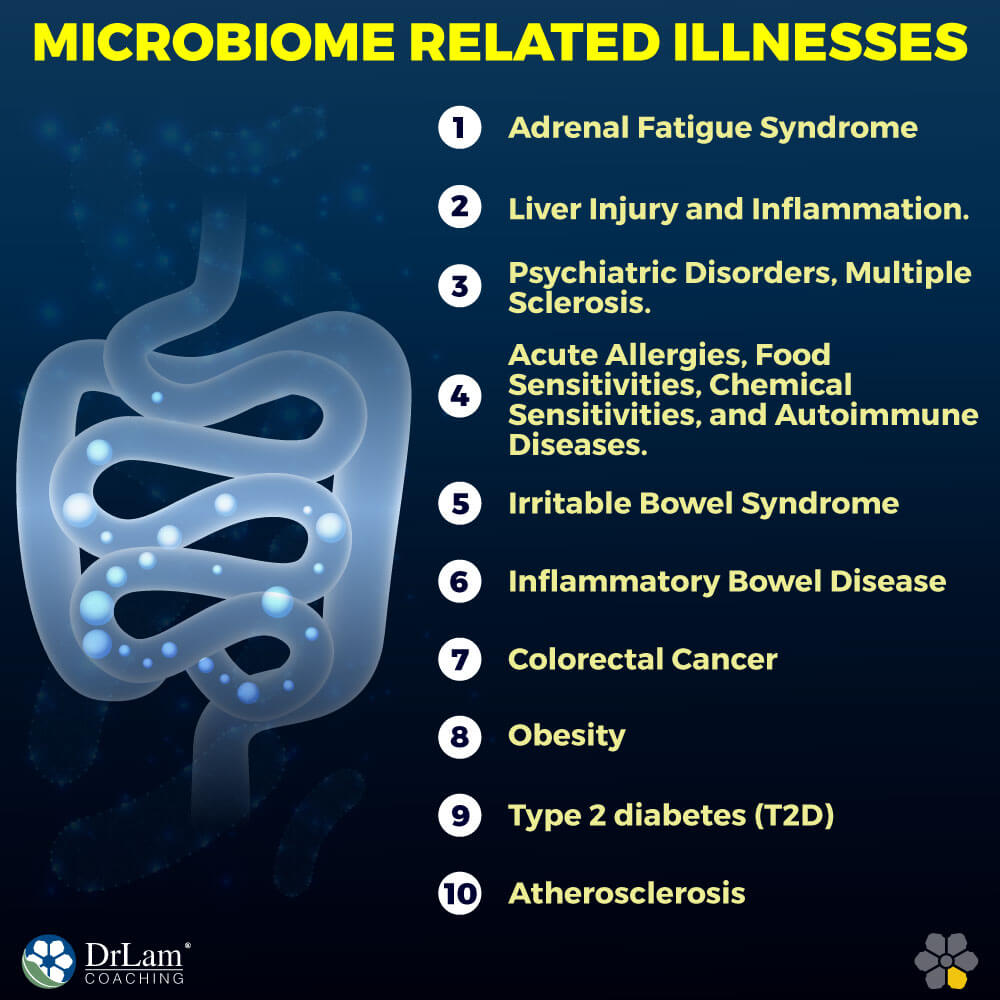
 Functional bowel disorderliness such as irritable bowel syndrome (IBS) is defined solely by symptom-based diagnostic criteria. Abdominal discomfort or pain as well as altered bowel habits are the main symptoms of the condition. The exact pathogenesis of IBS is likely a confluence of many factors; however, there seems to be a connection between alterations in gastrointestinal flora and irritating inflammation of the gut characteristic of IBS. The gut microbiome is also important in preventing pathogens from flourishing, so an altered healthy gut bacteria may also play a role in allowing colonies of disease-causing organisms to form.
Functional bowel disorderliness such as irritable bowel syndrome (IBS) is defined solely by symptom-based diagnostic criteria. Abdominal discomfort or pain as well as altered bowel habits are the main symptoms of the condition. The exact pathogenesis of IBS is likely a confluence of many factors; however, there seems to be a connection between alterations in gastrointestinal flora and irritating inflammation of the gut characteristic of IBS. The gut microbiome is also important in preventing pathogens from flourishing, so an altered healthy gut bacteria may also play a role in allowing colonies of disease-causing organisms to form.
Ulcerative Colitis (UC) and Crohn’s disease (CD) are both forms of inflammatory bowel disease (IBD). These conditions include recurring chronic inflammation of the gastrointestinal tract. They are, however separate and distinct, with differentiating patterns of symptoms. Crohn’s disease is thought to result from an interaction between the gut's microbe composition and the body's genetics. It is defined by chronic segmental gastrointestinal tract inflammation. The symptoms of ulcerative colitis are similar, being inflammation and ulceration of the lining of the colon. However, it is thought that these two conditions are not the result of a single pathogen, but arise from dysbiosis of the gut changing the gastrointestinal microbiome and altering the functional environment; although the exact causes remain widely debated. Studies have found gut microbes to be correlated with the development of IBD, and they are thought to be key factors in mucosal lesion formation. Despite the lack of a clearly defined etiology, the evidence points to a strong connection between gut microbial health and IBD.
The gut microbiome may also have a role to play in the development of colorectal cancer (CRC). Like IBD, no single pathogenic organism has been found for CRC, however some organisms of interest have been identified in studies. Fusobacteria was found in higher numbers in colorectal tumors in one study, suggesting a link between the two. These tumors are often causal of, or at least associated with, persistent and chronic inflammation in the gut, which is also a risk factor for CRC. The link between microbially induced inflammation and CRC has also been highlighted in some other studies. Indeed, it has been established that microbial products can enter barrier-defective colonic tumors, trigger inflammation through a host immune response and, in turn, increase tumor growth.
Obesity is a complex metabolic dysregulation that develops from a prolonged imbalance between energy intake and energy expenditure. Although lifestyle factors, diet, and exercise contribute largely to the modern epidemic, the gut microbiome plays an important part in the development of obesity.
 Many studies with both mice and human subjects have evidenced strong associations between changes in healthy gut bacteria and the development of obesity. Some studies have shown a flourishing of the microbial genera Firmicutes along with a corresponding drawdown of Bacteroidetes populations in the gut of obese subjects. However, this skewing of population ratios between different microbial families disappeared after weight loss, returning to those seen in the gut microbiomes of lean individuals. This skewed Firmicutes to Bacteroidetes ratio was also observed in mice subjects genetically predisposed to obesity.
Many studies with both mice and human subjects have evidenced strong associations between changes in healthy gut bacteria and the development of obesity. Some studies have shown a flourishing of the microbial genera Firmicutes along with a corresponding drawdown of Bacteroidetes populations in the gut of obese subjects. However, this skewing of population ratios between different microbial families disappeared after weight loss, returning to those seen in the gut microbiomes of lean individuals. This skewed Firmicutes to Bacteroidetes ratio was also observed in mice subjects genetically predisposed to obesity.
Genetic differences that can cause a predisposition to obesity include a greater number of genes coding for phosphotransferase functions that increase the rate of carbohydrate processing, and therefore suggesting it may be easier for these individuals to assimilate energy from sugar rich diets.
Using fecal microbiota transplantation as discussed above, studies have been conducted to introduce microbe populations from both obese and lean mice into mice with microbe-free guts. The results suggest a causal relationship between microbiome alteration and the development of obesity.
T2D is principally linked with obesity-related insulin resistance. However, several genetic and some environmental factors are thought to influence the condition. Alterations in the composition of the gut microbiome of adults with T2D in relation to that of healthy control subjects has been noted.
Incidence of type 2 diabetes (T2D) is rising in numbers parallel to obesity, and the environmental factors that are linked with T2D risk include diets high in carbohydrates and altered healthy gut bacteria. Low-grade inflammation is witnessed in T2D patients; diabetic mice and humans that have raised plasma levels of lipopolysaccharide (LPS), the membrane component of Gram-negative bacteria, which has been proven to impair glucose metabolism in mice. Germ-free mice have lesser macrophages in their adipose tissue and increased glucose metabolism compared with colonized mice.
Atherosclerotic plaques are hardened, thickened sections of the arterial wall resulting from accumulating white blood cells and adhesion of cholesterol to said wall. The thickening of the blood vessel wall is known as atherosclerosis, and it is often the cause of serious health events such as heart attack or stroke. Microbes from the genera Chryseomonas, Veillonella, and Streptococcus have been detected in these plaques, and they also exist in the oral cavity and the gut. Studies have demonstrated that patients who had experienced an atherosclerotic event had higher levels of Collinsella and lower levels of Eubacterium and Roseburia in their gut microbiome than healthy control subjects did.
 Mention the terms leaky gut, Small Intestine Bacterial Overgrowth (SIBO), food sensitivities, bloating, and autoimmune disease, and our mind seldom paints a picture of microbiome imbalance as a possible culprit. Yet it should be clear from the above discussion that the microbiome can play an important role in such disease pathology. While we are still in the early phases of research, evidence is mounting that symptoms associated with dysbiosis can reach far and wide, including symptoms that often appear unrelated to the microbiome. Unhealthy microbiome and adrenal fatigue have a variety of symptoms and vary among individuals. The variation in symptoms of unhealthy gut microbiome and adrenal fatigue makes diagnosis difficult. The symptoms include, but are not limited to,pain of unknown origin, rise of autoimmune conditions, food intolerance, food allergies, migraine headaches, blurry vision, metabolic slowdown, insomnia, depression, anxiety, and brain fog.
Mention the terms leaky gut, Small Intestine Bacterial Overgrowth (SIBO), food sensitivities, bloating, and autoimmune disease, and our mind seldom paints a picture of microbiome imbalance as a possible culprit. Yet it should be clear from the above discussion that the microbiome can play an important role in such disease pathology. While we are still in the early phases of research, evidence is mounting that symptoms associated with dysbiosis can reach far and wide, including symptoms that often appear unrelated to the microbiome. Unhealthy microbiome and adrenal fatigue have a variety of symptoms and vary among individuals. The variation in symptoms of unhealthy gut microbiome and adrenal fatigue makes diagnosis difficult. The symptoms include, but are not limited to,pain of unknown origin, rise of autoimmune conditions, food intolerance, food allergies, migraine headaches, blurry vision, metabolic slowdown, insomnia, depression, anxiety, and brain fog.
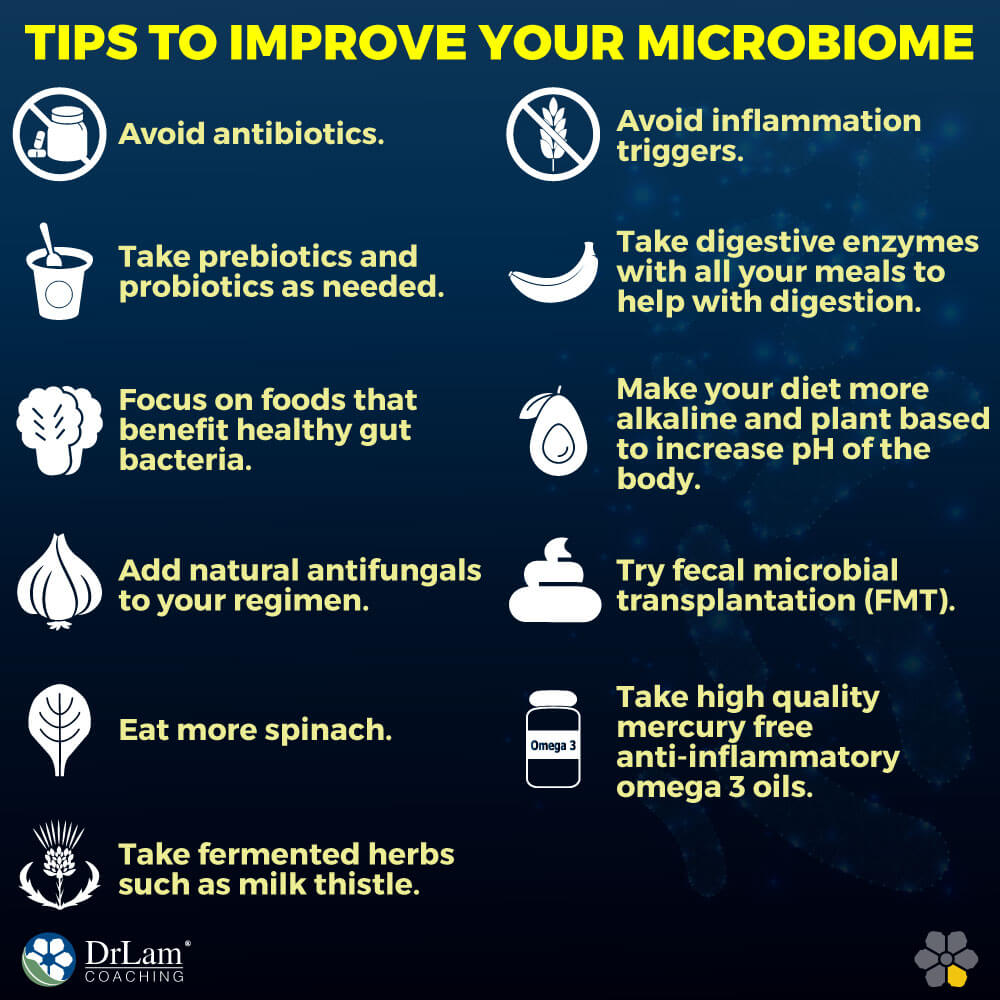
Here is the approach to regaining a healthier microbiome by reducing inflammation, improving assimilation, and restoring healthy flora.
 Eat more spinach. Fresh vegetables are an important source of good bacteria to aid in treatment of unbalanced gut microbiome and adrenal fatigue syndrome. The leaves of the spinach plant are estimated to harbor more than 800 different species of microbes. These are microbes that cannot be sterilized or removed from the plant by washing, because they’re actually inside the leaves. Therefore, having access to this kind of microbial diversity through the things that we eat is probably very important.
Eat more spinach. Fresh vegetables are an important source of good bacteria to aid in treatment of unbalanced gut microbiome and adrenal fatigue syndrome. The leaves of the spinach plant are estimated to harbor more than 800 different species of microbes. These are microbes that cannot be sterilized or removed from the plant by washing, because they’re actually inside the leaves. Therefore, having access to this kind of microbial diversity through the things that we eat is probably very important.Eating a diet high in refined carbohydrates, sugar, and alcohol can cause the candida population to grow out of control.
Fat cells have 4x more cortisol receptors than peripheral tissues.